
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recently identified coronavirus strain responsible for coronavirus disease 2019 (COVID-19) and the pandemic. SARS-CoV-2 emerged in China in December 2019 and is mainly transmitted via droplets and surface contact routes. Symptoms can be signs and symptoms of an acute respiratory illness such as fever, cough, shortness of breath, but the infection can also be asymptomatic. The virus infects human cells by interacting with the angiotensin-converting enzyme 2 (ACE2) on the surface of respiratory cells and spike(S) protein on the outer envelope of the virion particle, in particular with its receptor binding domain (RBD). The spike (S) protein and the nucleocapsid protein (NC) are the main immunogens of SARS-CoV-2. Antibodies against the RBD of the S protein are considered to have neutralising activity, as they block interaction with the ACE2 receptor and can thus block cellular infiltration. While the scientific community has been focusing on these neutralising antibodies for some time, the emergence of immune-evasive variants and the first studies to develop vaccines with the nucleocapsid of SARS-CoV-2 will create the need to identify both antibody species.
We therefore decided to use our FluoBolt™ technology to develop the first quantitative duplex antibody test (DAT) for antibodies against SARS-CoV-2.
The “Cov19 FluoBoltTM-DAT” (Art. No. FIA-1707-FC5) enables the simultaneous and quantitative determination of antibodies against the S1RBD AND nucleocapsid antigen of SARS-CoV-2 within a single measurement using only 10 μl of sample and provides results within 60 minutes.
In contrast to other antibody assays that detect all antibodies produced against the S1 or NC protein, the “Cov19 FluoBoltTM-DAT” is epitope-specific and detects immunodominant antibody species, i.e. the most important antibodies produced either by vaccination or infection. This is also very well demonstrated by the fact that the assay recognises anti-S1RBD antibodies against all dominant virus variants identified to date (see diagram below):
Therefore, the “Cov19 FluoBoltTM-DAT” assay can be a valuable tool to establish an antibody-based “protective correlate”, i.e. to determine which amount and type of antibody provides protection for a certain period of time.
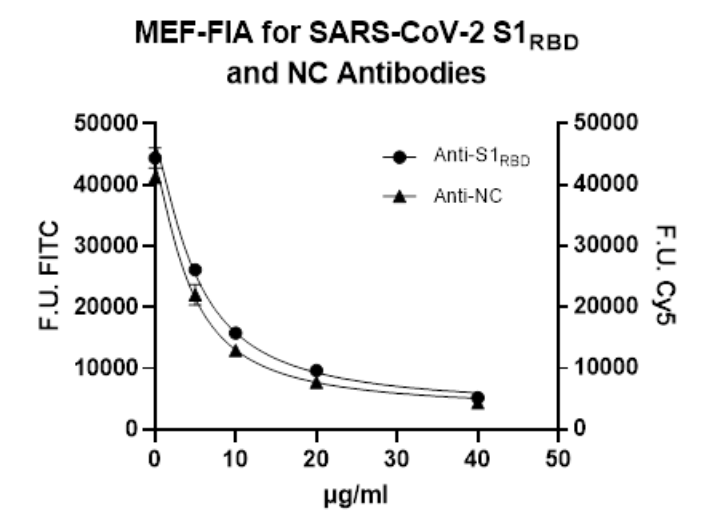
Anti-S1RBD and anti-NC antibodies present in serum or plasma samples from patients compete with analogue fluorescently labelled antibodies (FITC and Cy5-labelled) for the binding sites of the NC protein and the S1RBD domain coated on a Metal Enhanced Fluorescence-Microtiter Plate (MEF-MTP). There is a direct correlation between the amount of SARS-CoV-2 antibodies in the sample and the amount of fluorescence units (FUs) measured with a fluorescence microplate reader. Calibrators with known levels of anti-S1RBD and anti-NC antibodies are used to generate calibration curves to quantify the antibody concentration of an unknown sample.
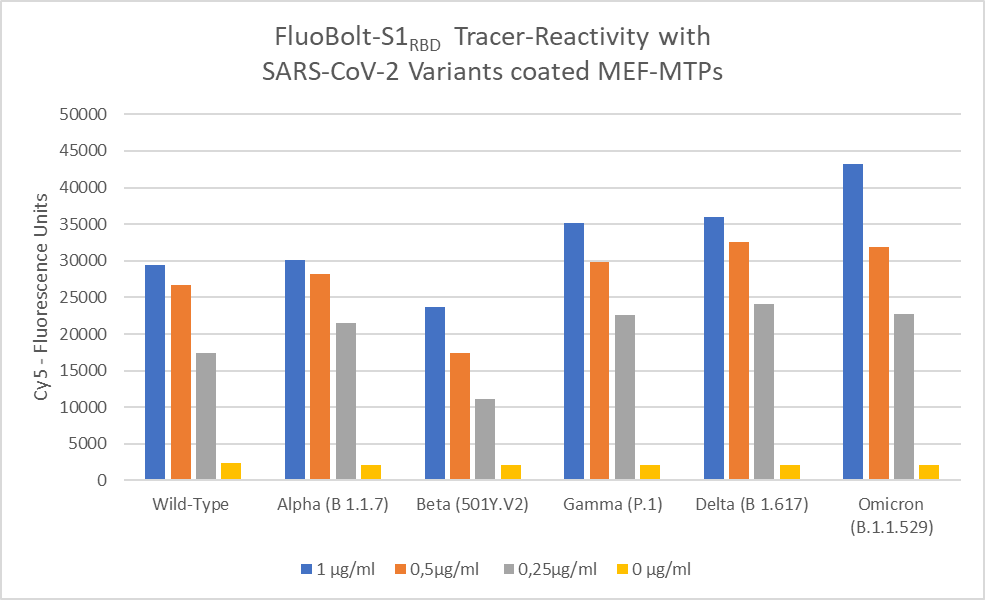
Impact of SARS-CoV-2 variant-associated RBD mutations on the susceptibility to serum antibodies elicited by COVID-19 infection or vaccination. Chen LL et al., Clin Infect Dis. 2021 Jul 26: doi: 10.1093/cid/ciab656. Online ahead of print.
Neutralizing antibodies against SARS-CoV-2 variants induced by natural infection or vaccination: a systematic review and pooled meta-analysis. Chen X et al Clin Infect Dis. 2021 Jul 24:ciab646. doi: 10.1093/cid/ciab646. Online ahead of print.
Antibody response to SARS-CoV-2 infection in humans: A systematic review. Post N et al., PLoS One. 2020 Dec 31;15(12):e0244126.
The Nucleocapsid protein triggers the main humoral immune response in COVID-19 patients. Smits VAJ et al., Biochem Biophys Res Commun. 2021 Mar 5;543:45-49
Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals.
Brochot E. et al., Front Microbiol. 2020 Oct 19;11:584251
The SARS-CoV-2 spike protein: balancing stability and infectivity. Berger I, Schaffitzel C. Cell Res. 2020 Dec;30(12):1059-1060. doi: 10.1038/s41422-020-00430-4. PMID: 33139926; PMCID: PMC7604330
Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020 Aug;584(7819):115-119. doi: 10.1038/s41586-020-2380-z. Epub 2020 May 26. PMID: 32454513.