
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a recently identified coronavirus strain responsible for coronavirus disease 2019 (COVID-19) and the pandemic. SARS-CoV-2 emerged in China in December 2019 and is mainly transmitted via droplets and surface contact routes. Symptoms can be signs and symptoms of an acute respiratory illness such as fever, cough, shortness of breath, but the infection can also be asymptomatic.
The virus infects human cells by interacting with the angiotensin-converting enzyme 2 (ACE2) on the surface of cells in the respiratory tract and spike (S) protein on the outer envelope of the virion particle, in particular with its receptor binding domain (RBD). The S and the nucleocapsid protein (NC) are the main immunogens of SARS-CoV-2. Antibodies against the RBD of the S protein are considered neutralising, as they can block the interaction with the ACE2 receptor and thus prevent cellular infiltration.
The emergence of more and more immunoinvasive and/or infectious variants and declining immunity after infection or vaccination creates an ongoing need for SARS-CoV-2 antibody measurements.
Therefore, to complement our SARS-Cov-2 serology portfolio, we have decided to utilise our FluoBolt™ technology to develop a
DUAL, QUANTITATIVE MEF-FIA ANTIBODY FÜR DIE BESTIMMUNG VON ANTIKÖRPERN GEGEN DAS NUCLEOCAPSID UND DIE S1-REZEPTORBINDUNGSDOMÄNE DES HUMANEN SARS-COV-2 VIRUS (“Cov19 FluoBoltTM-DUO SN” Art. No. FIA-1708-C5) zu entwickeln.
In contrast to our “Cov19 FluoBoltTM-DAT” (Art. No. FIA-1707-FC5), this new assay is not epitope-specific and therefore allows the quantitative measurement of a broad spectrum of antibodies against the S1RBD and the NC antigen of SARS-CoV-2 on separate plates.
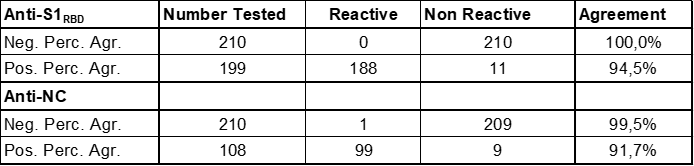
This results in excellent clinical agreement and correlation with many other assays used in SARS-Cov-2 serology:
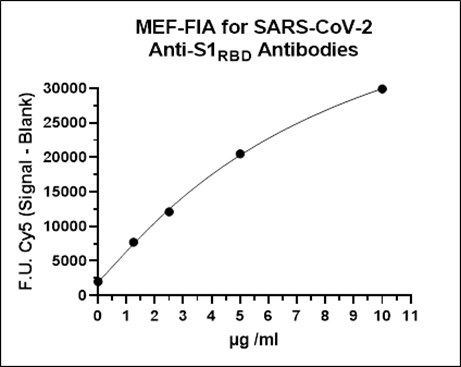
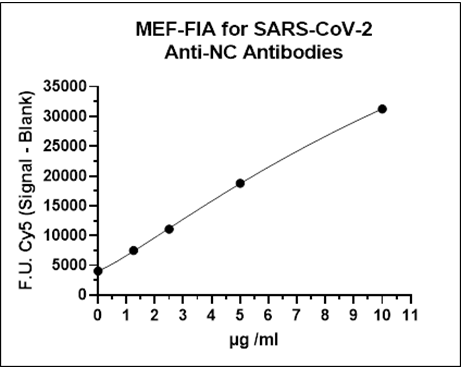
Anti-S1RBD and anti-NC antibodies present in patient serum or plasma samples bind to NC protein and the S1RBD coated on separate Metal Enhanced Fluorescence-Microtitre Plates (MEF-MTP). Bound antibodies are detected using Cy5-labelled anti-human antibodies. Calibrators with known levels of anti-S1RBD and anti-NC antibodies are used to generate calibration curves to quantify the antibody concentration of an unknown sample.
Antibody response to SARS-CoV-2 infection in humans: A systematic review. Post N et al., PLoS One. 2020 Dec 31;15(12):e0244126.
The Nucleocapsid protein triggers the main humoral immune response in COVID-19 patients. Smits VAJ et al., Biochem Biophys Res Commun. 2021 Mar 5;543:45-49
Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals. Brochot E. et al., Front Microbiol. 2020 Oct 19;11:584251
The SARS-CoV-2 spike protein: balancing stability and infectivity. Berger I, Schaffitzel C. Cell Res. 2020 Dec;30(12):1059-1060. doi: 10.1038/s41422-020-00430-4. PMID: 33139926; PMCID: PMC7604330
Human neutralizing antibodies elicited by SARS-CoV-2 infection. Ju B, Zhang Q, Ge J, et al. Nature. 2020 Aug;584(7819):115-119. doi: 10.1038/s41586-020-2380-z. Epub 2020 May 26. PMID: 32454513.
Application of SARS-CoV-2 Serology to Address Public Health Priorities. Sherman AC et al., Front Public Health. 2021 Nov 23;9:744535.
Silent SARS-CoV-2 Infections, Waning Immunity, Serology Testing, and COVID-19 Vaccination: A Perspective. Narasimhan M et al., Front Immunol. 2021 Sep 21;12:730404.
Remote Fingerstick Blood Collection for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Testing.
Garcia-Beltran WF et al., Arch Pathol Lab Med. 2021 Apr 1;145(4):415-418.
Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. Matta J et al.,JAMA Intern Med. 2022 Jan 1;182(1):19-25
© 2025 All Rights Reserved.